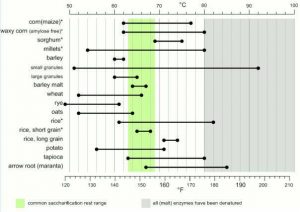
Left: December 26th, 2014 – I dug this picture. First one of me in a long while. (over 270 lbs). Right: September 2016 – Tasting my Saisons Also, how do I always forget to shave? – (~170lbs)
WARNING: You’re entering a really non-beer zone. What follows is an article I originally wrote about a year ago. Why post it now? Because it turns out this is exactly the time of year when everyone’s “I’m eating healthier. Going to exercise! Get in shape! It’s on like Donkey Kong” resolutions suddenly go out the window. So here’s your extra motivation – keep going – it’s possible! (FWIW – I’m still sitting at 170lbs – not too shabby!)
Our passion and pursuit of brewing knowledge and perfection comes at a somewhat heavy price – our waistlines. A great many homebrewers face the great modern epidemic of growing girth with a major disadvantage – namely, the extra calorie burden of a great tasting beer.
I’m one of them. Hi, my name is Drew and I’m a recovering fat person. I’m also the goof responsible for a lot of scribbling about beers both sublime (Pliny the Toddler) and ridiculous (Clam Chowder Saison!). In November 2014, I stepped on the scale at my doctor’s office and blanched in horror as the scale crossed the previously unsummited peak of 270 lbs. On my 5’10” frame that so ridiculously gargantuan as to make me laugh-cry. But here’s the sad part – that wasn’t enough. Even with years of wishing to change, worsening health and a holiday trip that almost saw me losing exit row seats because I could barely fit the seatbelt about the expanse of epidermis – I still didn’t change.
What finally kicked me down the road to a better me – getting sick. An epic two-week bout of Peruvian Guinea Pig Death Flu finally did it. On January 4th, 2015 I had my homebrew club meeting. Did my official educational song and dance, went home and promptly fell into bed. For two long miserable weeks I survived on a diet of warm chicken broth and Theraflu. Coming out the other side, I found my weight was at 260. That’s what it took. After that little bump, I wasn’t looking back.
But what about the beer? We’ll get there.
A Quick Note About All Things Dietary & Health

I am not a doctor, a registered dietician, nor an expert of any variety. I’m an engineer with an engineer’s obsession around numbers and formulas. This means I suffer from an engineer or physicist’s belief that everything can be neatly quantified and turned into a life changing equation. It’s a fool’s errand to create a catholic system because it’s impossible to fit everyone’s needs. What I’m going to describe here is what worked for me. In the words of my wife “I’m a stubborn mule”. Once I have a plan in my head, I get to work and keep going until I fall over.
My diet worked for me – it might not work for you. There are multiple ways to lose weight and different approaches work better for different people. And do I even need to remind you that making big health/lifestyle changes should be reviewed by an actual medical professional first? This goes double if you have any sketchy health issues. Remember, a diet is intentionally stressing on your body’s systems. Don’t play fast and loose with them!
How to Lose Weight in 4 Easy Steps
Here’s the core of what I did:
– Tracked my calories on my phone using the MyFitnessPal app
– Made sure the things I ate fit inside those limits.
– Walked 2-5 miles per day.
– Be consistent. Nothing screws you up as much as not sticking to your plan.
That’s it. I wish there was a magic trick or a pill or something, because if there was I could be as rich as Croesus. But no, the heart of the matter was coming down to the decision to stuff less junk in my pie hole and work the weight off that way. You’ll hear people refer to this as Calorie In / Calorie Out (CICO). It’s simple; it’s old fashioned and these days actually practical to do with a few simple tools.
Counting Calories – A How To

In the pre-smart phone era, if you wanted to calorie count it was tedious, extraordinarily so. These days with any one of a handful of apps, calorie counting is a simple matter of typing or taking a picture of barcodes. I used the MyFitnessPal app, which ties in with all sorts of health gadgets and other apps to help track the impact of your daily exercise. Literally to use MyFitnessPal, you just type in what you’re eating, add a recipe or scan a barcode. The app helps you calculate how much you should eat per day (calorically) and helps keep a record of how you’re doing.
But that’s not all you need – a scale, well two, are handy. One is your traditional scale. Shed the clothes as comfortable and weigh yourself. Do it consistently, every day or every week at the same time. I prefer pre-coffee, pre-breakfast, pre-morning angst because that’s going to be as low as I go for the day. Be consistent about it (a theme of this whole effort)
The second is a food scale of some variety. (This is the one I used)Using your hop scale is a great way to reset your picture of proper portions and you’re showing your partner that your brewery purchases can have greater utility. Why a scale? Because seriously – that scoop of delicious nutrition packed peanut butter? Many more calories than you think.
On the one hand, don’t worry about super precision down to the calorie level tracking – you’re playing a game of thousands, not ones. But on the other hand, measuring your food is important to help you know how to eat the correct amount.
Why not <Blank> diet?
These days lots of people swear by carb cutting. Low carb diets (ala atkins/keto/paleo) which work by short-circuiting the “normal” dietary metabolism in favor of ketosis. For some folks, these diets seem to work, but not for me so that’s why I didn’t use that approach.
I’m not a fan of restriction/elimination diets because I don’t feel they’re sustainable. Plus I get a little suspicious when a diet promises me no repercussions for all the bacon I can eat. At some point we all backslide and for many of the restriction diets, a backslide can throw your body out of the diet’s desired state of metabolical activity.
For many low carb diets that causes a pretty dramatic “instant” weight gain as the body creates new stores of glycogen from the influx of carbs. It gets bound with additional water for even more apparent weight gain. Plus let’s face it – from a brewer’s perspective – low carb and beer don’t play well with each other.
There are countless other diet plans out there like Glycemic Index diets that look to curb blood sugar changes by avoiding foods that drop a high amount of glucose into the blood stream or counter balancing carbs with relatively equal loads of protein. The diet basically works to curb hunger and over eating by preventing large swings in insulin production and blood sugar. Proponents also point out that the body will burn carbs before burning fat calories and when your goal is to drop fat, why interfere with it?
There’s still a lot of scientific debate rolling back and forth with different studies offering different evaluations of effectiveness, but again for some people GI diets work quickly and like a charm with other health benefits claimed.
Unfortunately, beer has a high glycemic index, which makes it difficult to incorporate with a GI approach without due diligence paid to balancing out the carb load. A fellow brewer swears by the diet, carrying cubes of chicken breast to competition to equalize his beer intake. He’s lost a bunch of weight following this plan, but even still when he’s in hardcore mode, he still prefers spirits for their lower hit. Remember – different diets work for different people!
One advantage of old fashioned dieting; if you backslide, you take your lumps and go right back to it. No keto flu, no carb cravings, etc. Just accept the “slip” and move on. Remember consistent application of principles will get you there.
What I Eat (Including Recipes!)

An Imperfect, but better than usual Valentine’s Day meal – sauteed spinach, seared scallops on a bed of orange cauliflower puree and a beurre blanc sauce – a little healthy, a little cheaty.
Important thing to know – I love to cook and I love to eat. So what the heck do I eat now that I no longer have a free hand with the olive oil jug and the cheese? Is it all just vegetables? No, but there are a ton more veggies in my diet now than used to be!
My daily goal is to get 50% of my calories from carbohydrates, 30% from protein and 20% from fats. Again, using a tracking app makes it super easy to know if you’re there. Important to note – as much as possible, I avoid large amounts of simple carbs aka refined sugar, sugar syrups, bleached flour, pasta (sob) and less complex breads. These have a tendency to make me hungrier possibly from blood sugar effects (see glycemic index above). I just don’t tend to feel as full or as happy after them so, nope. Also, let’s face it, I’m drinking beer and other fun beverages while dieting and that’s enough stupidity to not add to it.
I simplified breakfast by almost always eating the same thing. I make a breakfast sandwich based on a whole wheat English muffin, egg whites, a little cheese and some flavorful protein source like smoked salmon. Lunch is a salad or veggies with lean protein – aka chicken breast. Dinner is something tasty, but still light – my turkey chili; a sweet potato topped with tomato sauce, turkey Italian sausage and mozzarella, a veggie burger, a BLTA, fish, shrimp, etc.
My focus is almost always on a lean protein with a spicy condiment and a bunch of fibrous vegetables like kale or zucchini. I don’t have cholesterol issues so I eat a mountain of shrimp cocktail because fancy and tasty. Remember spices are your friends! Here are a few of my favorite palate saving recipes:
A Typical Diet Day:
6:30AM: Cold Brewed Coffee (about a pint. Don’t talk to me until consumed)
7:00AM: New Yorker McMuffin
10:30AM: Celery/Carrot Sticks/Sugar Snap Peas (or a protein bar on lifting days)
12:30PM: Slow Cooked Buffalo Chicken Wrap with salad
2:00PM: Greek Yogurt
4:00PM: Granola/Kind Bar/Turkey Jerky
6:30PM: 5 oz. roasted salmon filet, zucchini noodles, and sweet potato
8:00PM: Frozen Yogurt Bar
10:00PM: Chocolate Covered Almonds
12:00AM: Sleep
New Yorker McMuffin
~200-300 calories
1 Whole Wheat English Muffin (Double Fiber even better to stay full)
0.75 oz. goat cheese
2 thin slices yellow onion
A handful of capers
1 oz. smoked salmon
4 oz. Egg White Substitute, scrambled with cooking spray
Salt
Black pepper
Dried thyme
Squeeze sriracha, if desired
Pickled peppers, if desired
– Toast the muffin, spread with goat cheese.
– Top cheese on one side with half the onion, scatter the capers on there and top with salmon
– In a non-stick skillet, scramble the egg white and spices briefly before letting it set into a sheet. Fold it and let brown slightly.
– Add egg on top of the salmon, top with the other part of onion and pickled peppers, if desired
I also do this with Canadian bacon or turkey bacon or turkey sausage and cheddar (1/2 oz.) and no capers for a variation
Chicken Sausage Kale Bowl

~300-400 calories
This is tasty and good for you, plus the kale does a great job filling you up. Shut up, I know Kale. Give this a shot – you’ll actually enjoy it.
1/2 medium yellow onion, diced
2 cloves garlic, minced
1-2 links Chicken Sausage, diced
5-6 oz. cut, cleaned Kale
Splash olive oil
Splash balsamic vinegar
1 egg, sunny side up
1/2 oz. grated Parmesan
– In a skillet, spray with a little olive oil mist. Add onion with a pinch of salt – sweat the onions until soft. Add the garlic and stir until fragrant. Add the chicken sausage and brown.
– Add the kale to your skillet, stir it around to get coated and then cover the pan and let the kale wilt for 5-10 minutes or until soft, but not mushy. It should have wilted into a dark green tangle
– Add the olive oil and vinegar and toss the kale to coat.
– Serve in a bowl topped with the egg and Parmesan and some hot sauce to boot.
Zucchini Turkey Sausage Lasagna

Makes 6 servings in a 9×13 pan – because they lie when they say “8 servings” No one eats 1/8th of a 9×13 pan of lasagna without feeling a pang of regret and nibbling off small pieces with a fork.
Approximately 420 calories per serving. (Compare that to traditional lasagna which has something like eleventy billion per slice – and you eat two of them)
“Noodles”:
3 largish zucchini, sliced into planks on mandoline, salted liberally on paper towels. I use the second thickest setting on the mandoline to get sturdy slices that don’t fall apart but also don’t dominate the meaty cheese goodness.
Sauce:
1 onion, diced
4 cloves garlic, minced
3 links hot Italian turkey sausage
1 tbsp. tomato paste
10 oz. crimini mushrooms, chopped
1 big can crushed tomatoes
2 oz. vermouth/wine
1 tsp oregano
Cheeses:
12 oz. part skim mozzarella, shredded
12 oz. ricotta
1 egg
1 oz. grated Parmesan
Instructions
Preheat oven to 350F
Noodle Prep
Prep zucchini and let salt do it’s magic for ~20 minutes while you assemble the sauce.
Sauce Prep
Sweat onion over medium-high heat with a little olive oil (or spray oil) in your saucepot with salt. Get translucent. Add garlic, stir until fragrant with salt. Remove sausage from casing; break up into wee little pieces in the pot. Sauté until no longer pink. Add tomato paste to pot and stir until no longer bright red and threatens to burn. It’s flavor, shut up. Add chopped up mushrooms with so much salt. Cook for 10 minutes until mushrooms release moisture and moisture goes bye bye. Add tomatoes. Rinse can with wine and add to pot. A little booze actually helps release flavors otherwise not available. Simmer until thick – 20-30 minutes.
Noodle Cook
Now that zucchini has been sitting a while, dry with paper towels. Place onto baking sheets lined with silicone mats or parchment paper. Bake for 15 minutes. Pull and allow to cool.
Lasagna Assembly and Cookery
Mix the ricotta and egg together with some salt and pepper. Set aside
Take a 9×13 pan, I use an enameled cast iron because heavy is good. Add a layer of sauce to bottom of pan. Lay in half the “noodles” and top with more sauce. Add most of the ricotta mixture. Spread it around. Add 1/2 the mozzarella.
Layer in more noodles. More sauce, the last bit of ricotta, the other half of mozz and then sprinkle with the Parmesan on top.
Put 9×13 pan on a cookie sheet and bake for 35 minutes in the middle of the oven. Turn the broiler onto low and broil for 5-7 minutes until the top is so, so gloriously brown.
Pull for the oven, wait 15 minutes to avoid burning the hell out of your mouth.
Turkey Three Bean Chili

Makes 8 giant servings – roughly 350 calories per serving (without cheese or other condiments)
1 large yellow onion, diced
6 cloves garlic, minced
1 container extra lean ground turkey (20 oz.)
2 tablespoons cumin powder
2-3 tablespoons chili powders (I mix several homemade together)
2 cups water
2 oz. Tequila
28 oz. can Crushed Tomatoes
15 oz. can Fire Roasted Tomatoes
15 oz. can Lower Sodium Pinto Beans
15 oz. can Lower Sodium Black Beans
15 oz. can Lower Sodium Red Kidney Beans
14 oz. smoked Turkey drumstick, meat stripped and roughly chopped
Cilantro to taste
– Spray down a large Dutch oven and heat over medium heat. When hot, add the onion and a big pinch of salt. Stir and cooking until onion is translucent. Add garlic and allow to get fragrant – 30-60 seconds.
– Add the ground turkey, stirring to break up into small pieces. (With meat this lean, I prefer small pieces to avoid a tough chew). 5-10 minutes
– Once the turkey is no longer pink, add the spices and heat them through in the turkey juices. Add 1 cup of water and let the water bloom and boil – turn your spices into a spice slurry. Repeat with the second cup of water and then finally with the tequila. Your pot should be filled with an aromatic punch and a bunch of turkey and onions.
– Reduce the heat to low and stir in the tomatoes, beans and smoked turkey. Add a few roughly torn up sprigs of cilantro. Let simmer for 45 minutes or until the tomatoes no longer taste “tomatoey” and the chile and cumin flavors shine through
How to Eat – Snacks and On the Run
A good thing to figure out about yourself is how you like to eat. Turns out I like to have constant stimulation and so for me it worked out that I preferred having the ability to grab a snack on regular basis along with smaller main meals. Gotta track the calories though!
Snack Foods
– Turkey Jerky
– Nuts (I particularly love chocolate covered almonds – careful though nuts are calorie dense)
– Greek yogurt with some variety of fruit mix in
– Carrot/Celery sticks/sugar snap peas
– Water crackers and cheese/hummus/nut butter/yogurt spreads
On the run eating or eating out is another challenge because we don’t always have kitchen access at the ready. My best plan for eating out has always been to make up my mind before hand. In other words, say unto myself before entering that restaurant “we’re going to look for a nice piece of fish or chicken and ignore the pizzas and pastas that we want to bury our face into”. That little mental exercise is enough to give me the extra mental clarity to make a more dietically friendly choice. It’s not perfect – sometimes the gooey nature of an artichoke spin dip overrides my sense, but that’s the way it rolls.
The other important tip about eating out – don’t eat all of it. Restaurant portions are insanely huge in this country – usually 2-3 times appropriate single meal sizes. Some people ask the waiter to box half the meal when they order, but my sense of social propriety makes that untenable. Instead, eat slowly and sensibly, stop and let your brain catch up to the body’s signals of satiety.
Beer on a Diet

Here’s the part you wanted to know – can you drink on a diet? Turns out that the answer is yes, but carefully. I limited my drinking for the first two months of my diet. Part of it wasn’t hard – I still wasn’t feeling well. I pretty much only drank at club functions and even then didn’t drink at our board meetings. Slowly, I incorporated more drinks over time as I felt better and fitter. But – most importantly, I fit the beers into my daily calorie count.
Take a look at that “daily diet” day. On days when I’m at a club meeting or festival, the number of snacks decreases. The meals become leaner – chicken instead of salmon. More veggies and skip the sweet potato and dessert. That’s how I do it.
The hardest part about drinking – avoiding two voices – the voice that says, “You know what sounds like a lovely idea? Another drink!” and the other voice that says “hey, I could really go for a large double cheese double meat pizza.” The first, the “grog monster”, is easy to avoid if you can make a plan and stick to it.
The second is harder because that voice is your body’s natural reaction to drink induced blood sugar drops. In a nutshell – alcohol consumption causes fluctuations in insulin and blood sugar levels. When your blood sugar crashes thanks to increased insulin, your brain fires off signals that you receive as an emergency bat signal for immediate food consumption – the more caloric, the better and naturally the worse for your goal of losing weight. If drinking is taking to excess, this can get rather dangerous, but for your garden variety having a couple of drinks – don’t listen to it or at least grab a healthy snack instead of that giant tray of mini bagel pizzas you see.
But, but… looking at the Internets many craft beers fall into the realm of 200-300+ calories per serving. How the heck am I going to fit all of those into my daily regimen and still eat food? A strategy you can use, and many people do this to incorporate “cheat days” is to use “intermittent fasting”. Plan ahead – if you know you’re going to a beer festival on Saturday, increase your calorie deficit during the week by 100-200 calories to give yourself a little extra room on Saturday. You’re basically lending yourself calories to use. Remember what I said about cheat days? Well, there you go – beer days are my cheat day – kinda.
Oh and be careful! As you lose weight, the amount of alcohol you can safely consume (in terms of intoxication) drops. On the one hand, your bar tabs will go down since you’re no longer downing four pints of 10% beer. On the other, you’ll need to keep a better eye on yourself and remember things like cabs, Uber/Lyft and designated drivers. Heck, wouldn’t hurt for you to be the DD for your friends for a bit!
Exercise – How, What, When and Why Not to Push It

The Only Gym Selfie I’ve Ever Taken – As Far As You Know
Astute readers will notice I’ve only said one word about exercise and that’s “Walked”. Most people desirous of weight loss go all in. They get gung ho about their resolution to lose weight and start dieting and hitting the gym at the same time. Gotta burn up those calories after all and make up for lost time.
It’s a natural reaction, but I feel like it’s a formula for failure. Why? At least for my personality, this whole weight loss thing had to start with me re-training my food habits that trying to add exercise to it would have been overwhelming.
Exercise is good and it’s a key to getting truly healthy. Adding exercise, particularly strenuous cardio, will burn calories, but it also will ramp your appetite at a time when you have to deal with the first throes of “Can’t eat all the stuff I used to eat” syndrome. That’s sounds like a perfect storm of “I want to eat” desires at a time when you’re trying to ignore them.
After my first month of dieting, I started simple. I took my army of Chihuahuas on a daily walk. At first it was just around the block, every day before work. At work, I walked around my office building at lunch. Gradually, I added distance. The morning walk grew to 2.5 miles with some very exhausted dogs happy to sleep for the day. The work time walk grew to encompass the whole lunch break pushing up to 4 miles. Did the dog walk every day. Longer walks 4 times a week. Eventually I even bought a pair of proper shoes to do a little light running. For the vast majority of this project, this is all I did for exercise.
Depending on your physical condition, exercise can put you in a worse position from a weight loss perspective. You go for a jog, twist an ankle, get discouraged and then start to backslide. If you’re not prepared for it, the backslide can become permanent.
A mental obstacle I tripped myself up with for years is “exercise reward” thinking. That’s the little voice saying, “Dude! You rocked that treadmill today! You crushed those weights! You must have burned eleventy thousand calories, look at the machine’s counter! You deserve a reward of a nacho pizza cake!” (Goggle “nacho pizza cake” to shake your head at humanity’s waist)
It’s a fool’s errand to listen to that voice. He’s wrong for several reasons. First, no matter how many sensors you’re using, anything telling you how many calories you’ve burned is going to be woefully inaccurate and can lead you into the delusional garden of exercise eating.
Secondly, Penn Jillette, the loquacious half of Penn & Teller, lost a bunch of weight recently and developed the motto of “You can’t outrun your mouth”. While I disagree with some aspects of his diet plan (which is extremely low calorie), I whole-heartedly agree with his motto. Unless you’re training like Michael Phelps, exercise alone isn’t going to compensate for a diet of Double IPA, double cheeseburgers and double pieces of pie.
Having said all of that – as I approached my final goal weight – 170 – my weight loss slowed. Gone were the days of losing 2 lbs. a week while eating 1900+ calories. I needed more exercise and that’s when I think you want to bring in the big guns, the strength training. Adding muscle does wonders for keeping calories at bay. It also requires more calories, preferably “clean” calories to keep building, which is what makes it tricky – more food means more possible weight if you stop working out.
Now my weekly routine consists of a full body strength routine three-four times a week with a circuit training class plus walking/jogging the other days. When I’m in the gym I simply do squats, overhead presses, deadlifts, rows, lunges and bench presses. That’s enough to get you started on the right course! Every day I try to do a little something for the mechanical system of my body.
How My Diet Went
Here’s the breakdown so you’ll know what to possibly expect. You’ve seen the front photo. I’ll drop some additional progress photos after this.
In my first month of dieting, including the time I was sick – I dropped 22 pounds. It’s insanely fast, but that’s the body getting rid of the excess water as well as fat that you’re carrying. It’s easy and amazing to watch that happen with just a little focus – but beware the “woo hoo! celebrate” messages in your head. As time went on, the monthly totals dropped – as they should. The second month, I lost another 12 pounds. By month five I was down nearly 60 pounds – over half way to my goal.
Actually, here’s a word about goals. I started this diet at 270+. My initial goal – the marker I set my sights on – was 200. Let’s get there! As time went, I adjusted my goal when I saw that I could still do it and had room to improve. Eventually 200 became 190; 190 became 174 to get to a healthy BMI (Body Mass Index – a ratio of height to weight) target for my height and then finally 174 became 170 because even numbers rock. Now my goal is to stay around there and enjoy life while being in a better space physically and health wise!
Eventually, I got to my healthy BMI target (174) in October – 9 months after starting. It took another two months after that to reach my final goal of below 170. (100 lbs down!) It happens.
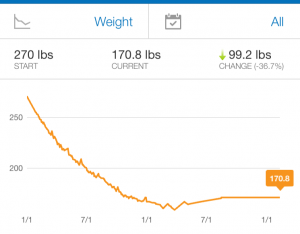
If you look at the graph of my weight loss, you’ll see that flattening of the scale. At first it’s easy to lose weight because your body needs a lot of calories. When I first started at 270, I could lose weight eating close to 2000 calories a day – that’s a lot of healthy food! Before I got more exercise incorporating, my restricted target was closer to 1500 calories per day – a much tougher goal.
Those bumps in the graph – those are completely normal. Some are tied to events like going wine tasting, visits from family, the AHA conference or travelling, etc. Some aren’t. Some are just the normal day-to-day fluctuations of your body mass. Don’t let an upswing get you down – particularly if you’re exercising or having a respite from the diet, but don’t get complacent!
Here’s an important tip if you have a long slog ahead of you – take some pictures. You’ve seen mine in this column showing my face. There’s a set of pasty puffy white shirtless dude as well – but those aren’t releasable on the orders of the Federal Government for the safety of society. Additional things to track – particularly when you get to the end of your journey and things become less about the number on the scale and more about other changes – get a measuring tape and learn how to take tailor measurements. You’ll be surprised at how many inches seem to disappear even though your weight stays the same. (Hello tricky fact that muscle mass weighs more than fat!)

February 2015 – 1 month in

April 2015 – 3 months in

June 2015

August 2015

October 2015 (I couldn’t fit into this shirt when the guys in Brazil originally gave it to me!)

December 2015 – Almost 1 year since the “good” photo
Life on a Diet
Back to our most important lesson – a diet as a “period of weight loss” is a terrible concept. It dooms us into this world of privation where we see a ticking countdown clock or a finish line just ahead that welcomes us back to a land of plenty. If you’re like me, being in a land of plenty with no restrictions is what got me into trouble in the first place. Instead, let’s focus on “diet” as a means of eating in a healthy fashion.
This also means you have to remember to live life, even while dieting. During my weight loss quest I went on several brewery trips, went to Brazil where I gave myself the meat sweats and drank cacahca and capharinas in the jungle, epic wine tastings, holiday meals, conferences, etc. Probably should have enjoyed a few more special meals with my family, but that’s my stupid laser beam focus kicking in. Try to live your life and recognize that things are meant to be experienced not just lived “correctly”.
Please remember, you’re bound to screw up. You’re going to have that extra beer, ice cream, pizza, nachos, duck fat fried French fries with aioli, etc. Then, if you’re like me, your brain will kick you in the metaphorical shins with demotivational words about your loserdom, which naturally activates that “sad eating” impulse. Take a page from the recovering folks at Anything Anonymous (hey, there’s an Overeater’s Anonymous!) – this is a one-day at a time effort. Leave the guilt in the confessional and keep working your program! You’ve got years to screw up and correct your mistakes!
Life Post Weight Loss
The dramatic part of my weight loss journey stopped in November of 2015 when I hit 165 lbs., down from my initial weight somewhere north of 270. In that time, I went from squeezing into size 44 “relaxed” jeans (at a waist size in excess of 50”) to “slim” size 32’s (waist- 35”) and from super tight XXL shirts to mediums. The truly terrifying number – I went from over 50% body fat down to ~18% – aka back into healthy range! Over time I’ve added muscle and dropped the body fat down to 14%. Currently, I’m up a little (172ish) because I’ve been injured and out of the gym, but we’ll correct that soon!
I still track my calories because it’s good to keep things honest and understand my caloric needs and how I’m doing.
And here’s the thing – this time is the hard part. Losing weight was annoying, but exciting and motivating as I could see progress before my very own eyes. There were plenty of congratulations being passed around once I lost 40 lbs. and people decided it was ok to comment and praise me. Those changing wardrobe sizes felt rad everywhere except my wallet. Even writing this article is a bit of an ego boost.
Now I’m in a world where the praise doesn’t come easy and the fight is one to maintain stasis. It’s not as sexy and rewarding and it’s a longer slog. But here I am now and I intend to stay somewhat here. I’ve set a line in the sand – if my weight moves back above a certain threshold, it’s back to business. Until then, just gotta keep fighting and resisting the seductive draw of the “easy” – the pizza, the cheeseburgers, all the fried goodies and “ahh, screw it, I’m too tired to cook let’s eat out”.
Let’s make a deal shall we? Let’s see where we’re at in a year and congratulate each other then for our success in either losing or maintaining. Just remember, we may not get to all the beers this year, but we’ll be in better shape to get the beers of the future!